CP-NET Clinical Database Platform: Genomic Architecture of CP (Whole Genome Sequencing)
|
The CP-NET Clinical Database recruits children born in 2009 or later with a confirmed diagnosis of cerebral palsy (CP) and contains data on clinically relevant risk factors, neuroimaging information, and neurodevelopmental functioning including psychosocial and participation outcomes.
This released dataset has whole genome sequencing data (WGS) on 221 children and youth with CP and their biological parents (656 samples). WGS was performed on DNA extracted from saliva on the Illumina HiSeq X platform, and the resulting CRAM files are provided. |
| Version: 1.0 | Date Released: On publication | Participants: 221 | |
| Files: 656 | Size: 46 TB | ||
| DOI: 10.60955/fszr-5q79 | ARK ID: | ||
|
Conditions: Cerebral palsy Modalities: Genomics Formats: CRAM |
|||
For further information
Comprehensive Whole-Genome Sequence Annotation to Elucidate the Genomic Architecture of Cerebral Palsy
Fehlings DL, Zarrei M, Engchuan W, Sondheimer N, Thiruvahindrapuram B, MacDonald JR, Higginbotham EJ, Thapa R, Behlim T, Aimola S, Switzer L, Ng P, Wei J, Danthi PS, Pellecchia G, Lamoureux S, Ho K, Pereira SL, de Rijke J, Sung WWL, Mowjoodi A, Howe J, Nalpathamkalam T, Manshaei R, Ghaffari S, Whitney J, Patel RV, Hamdan O, Shaath R, Trost B, Knights S, Samdup D, McCormick A, Hunt C, Kirton A, Kawamura A, Mesterman R, Gorter JW, Dlamini N, Merico D, Hilali M, Hirschfeld K, Grover K, Bautista NX, Han K, Marshall C, Yuen RKC, Subbarao P, Azad MB, Turvey SE, Mandhane P, Moraes T, Simons E, Maxwell G, Shevell M, Costain G, Michaud JL, Hamdan FF, Gauthier J, Uguen K, Stavropoulos DJ, Wintle RF, Oskoui M, Scherer SW (2024). Nature Genetics.
RECOVER: REaching patients with a COncussion Visiting the Emergency Room to enhance care
|
The Concussion Ontario Network: Neuroinformatics to Enhance Clinical care and Translation (CONNECT) is a collaborative network of 10 clinical sites across Ontario that aim to ensure new knowledge of concussion is translated into better diagnosis and care for the benefit of all Ontarians...
|
| Version: 1.0 | Date Released: 2023-12-01 | Participants: 16 | |
| Files: 672 | Size: 5.21 GB | ||
| DOI: 10.60955/qkh0-cx58 | ARK ID: 70798/d7hk4spx56tjf88fhf | ||
|
Conditions: Persistent post-concussive symptoms Scans: fMRI (resting state), DTI, T1W, ASL, SWI, FLAIR Timepoints: Baseline, Follow-up (Week 2, Week 4, Week 12) Modalities: concussion, PPCS, follow-ups, mood, pain, balance, reaction, sleep, symptoms, abilities, quality of life, treatment, neuroimaging, MRI, locomotion, saccadometry Formats: BVAL, BVEC, CSV, DOCX, JSON, NIfTI, PDF, XLSX |
|||
Ontario Neurodegenerative Disease Research Initiative (ONDRI): Foundational Study Longitudinal Data - Release 2.0
|
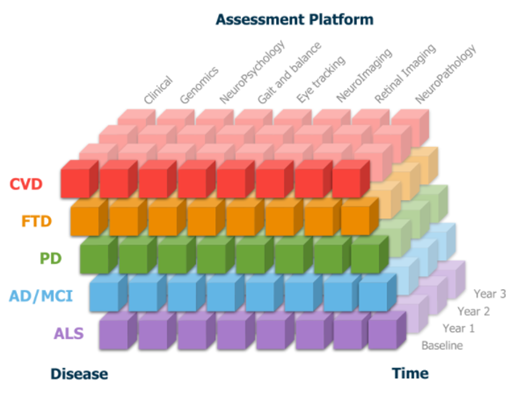
ONDRI's Foundational study includes data from 520
people living with Alzheimer’s Disease, Mild Cognitive Impairment,
Parkinson’s Disease, Amyotrophic Lateral Sclerosis, Frontotemporal
Dementia, or Cerebrovascular Disease, along with their care
partners...
|
| Version: 2.0 | Date Released: 2023-10-01 | Participants: 520 | |
| Files: 889 | Size: 365 GB | ||
| DOI: 10.60955/ygbs-na17 | ARK ID: 70798/d7r439px587bx4v6vq | ||
|
Conditions: Alzheimer's disease, Cognitive impairment, Parkinson's disease, Amyotrophic lateral sclerosis, Frontotemporal dementia, Unspecified cerebrovascular disease Timepoints: Screening, Baseline, 6 Month, Year 1, 18 Month, Year 2, 30 Month, Year 3 Modalities: Cohort study, clinical, Cognitive, Gait and balance, Eye tracking, Retinal imaging, Genetic markers, Neuroimaging, Neuropsychological assessment, Cross-disease, Cross-platform, Caregiver, Deep pheotyping Formats: CSV, TXT, NIfTI |
|||
For further information
Characteristics of the Ontario Neurodegenerative Disease Research Initiative cohort.
Sunderland KM, Beaton D, Arnott SR, Kleinstiver P, Kwan D, ,Lawrence-Dewar JM, Ramirez J, Tan B, Bartha R & Black SE (2022). Alzheimer's & Dementia.
Additional Resources
| ONDRI’s Research Initiative | ONDRI's Data Standards | Data Standards and Processes Videos |
Integrated Biological Markers for the Prediction of Treatment Response in Depression (Updated!)
|
The Canadian Biomarker
Integration Network in Depression (CAN-BIND) is a national
program of research and learning. From 2013 to 2017, data were collected from 211
participants with major depressive disorder and 112 healthy
individuals with an average age of 35 years...
|
| Version: 2.0 | Date Released: 2023-03-10 | Participants: 309 | |
| Files: 14990 | Size: 159.55 GB | ||
| DOI: 10.60955/mnwq-sq07 | ARK ID: 70798/d7v9bn9dj0pkm2rb7j | ||
|
Cohorts: Major Depressive Disorder, Healthy Controls Scans: EEG (resting state), fMRI (resting state), DTI, T1W, PDT2 Tasks: Emotional Face Categorization/Conflict Task (fMRI), go/no-go task (fMRI), anhedonia task (fMRI) Timepoints: Baseline, Phase 1 (Week 2, Week 4, Week 6, Week 8), (Phase 2 coming soon) Modalities: fMRI, Structural MRI, Diffusion tensor imaging, Electroencephalography, Genomics, Metabolomics, Methylation, Inflammatory markers Formats: BVAL, BVEC, CSV, EDAT, EDF, FDT, JSON, NIfTI, SET |
|||